american academy of pediatrics covid vaccine under 12
NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18.
Pfizer Says Covid 19 Vaccine Works In Kids Ages 5 To 11 Los Angeles Times
2 days agoAccording to the recent state-level data report published by the American Academy of Paediatrics AAP the pediatric population accounted for only less than 026 of all coronavirus disease-2019 COVID-19 deaths while eight states did not report any child deaths.

. Lee Savio Beers in a statement. The American Academy of Pediatrics AAP is urging the US. 5 to 11 and just over half of 12- to 17-year-olds were fully vaccinated according to.
The American Academy of Pediatrics AAP Advisory Committee on Immunization Practices ACIP Centers for Disease Control and Prevention CDC and the North Dakota Department of Health NDDoH all recommend COVID-19 vaccination of adolescents. American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12. While the Covid vaccines in use in.
AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines. Pfizer asks FDA to allow COVID-19 vaccine for kids under 5. American Academy of Pediatrics urges FDA to approve COVID vaccines for children under 12 Sep 13 2021 640 PM EST.
The American Academy of Pediatrics said the rise of the Delta variant changes the risk-benefit analysis for authorizing vaccines in. The vaccines safely help you build protection from COVID-19. Coronavirus disease 2019 COVID-19 school closures and quarantines have had substantial impacts on students health and education123 Clinical trials have shown severe acute respiratory syndrome coronavirus-2 SARS-CoV-2 vaccines to be safe and efficacious for adults adolescents and young children4 However in some areas vaccine uptake has been.
The American Academy of Pediatrics is urging the Food and Drug Administration to approve a COVID-19 vaccine for children under the age of 12 as soon as possibleWhile 70 of eligible Mainers 12. Editors noteFor the latest news on COVID-19 visit httpbitlyAAPNewsCOVID19. The dose may be different for younger ages.
Only the Pfizer-BioNTech Covid vaccine has been authorized for adolescents and teens ages 12-17. In addition to approving the vaccines use for adolescents on Wednesday the Centers for Disease Control. A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after the second dose of vaccine.
Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up. As Covid cases among children continue to rise in the US due to spread of the Delta variant experts are urging the federal government to fast-track vaccine approval for those under the age of 12. John Ionnidis estimated that the survival rate of the virus is 99986 percent for people between 20 and 29 years old and no lower than 997 percent for people.
Moderna has begun testing its COVID-19 vaccine in children the company announced Tuesday. 1 As COVID-19 continues to disrupt normal life with its continuous waves of infection the. Pfizer says itll put in the formal request for emergency authorization for kids 5 to 11 soon and the FDA could make its decision in just a few weeks.
That share may continue to climb as adults and children age 12 and older receive doses of the. Have resulted in death according to the American Academy of Pediatrics. The Food and Drug Administration FDA.
The company plans to enroll 6750 children ages. The AAP recommends adolescents ages 12 and older get vaccinated against COVID-19 now that federal health officials have signed off on the Pfizer-BioNTech vaccine for these ages. COVID-19 Vaccine for Adolescents 12-17 Years Old.
The AAP recommends against giving the vaccine to children under 12 years until authorized by the FDA The FDAs licensure of the vaccine which will be called Comirnaty applies to ages 16 years and older the group for whom emergency use authorization EUA was granted in December 2020. The dose may be. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older.
Earlier in May a child accounted for one in five coronavirus cases nationwide according to the American Academy of Pediatrics. Children have started receiving doses of mRNA vaccine as part of a phase 23 study called KidCOVE. American Academy Of Pediatrics Urges FDA To Approve COVID Vaccines For Children Under 12.
To get the most protection get all recommended doses of a COVID-19 vaccine. Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12. The viral vector vaccine in the United States is given in one dose to people 18 years and older.
Across the US children now represent more than 14 of all confirmed COVID-19 casesa percentage that has grown over the past month. Ad Safety is CDCs top priority. Now this comes as the delta variant significantly increased hospitalizations of children.
Stanford University medical professor Dr. PBS Newshour Posted on. While questions may now arise about the administration of the vaccine off-label for children aged 11.
Between 000-001 percent of all child coronavirus cases in the US. Kids under 12 could be one step closer to a COVID-19 vaccine shot. The vaccine will continue.
Last summer the American Academy of Pediatrics AAP advised against off-label use for children under the age of 12 said 2021 AAP President Dr. NPR As. FDA authorizes COVID-19 vaccine for adolescents ages 12-15.
Tuesday September 14 2021 Back to WASHINGTON DC.

Fda Again Warns Parents Not To Get Children Under 12 Vaccinated Yet The New York Times
Fda Pfizer Delay Authorization For Covid Vaccine For Kids Under 5 What To Know Cnet

Kids Covid Vaccine Side Effects What To Know
What Parents Should Know About Covid 19 Vaccines For Kids Under 12

What To Know As Covid Vaccines For Kids Under 12 Face Final Hurdles Nbc Chicago
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
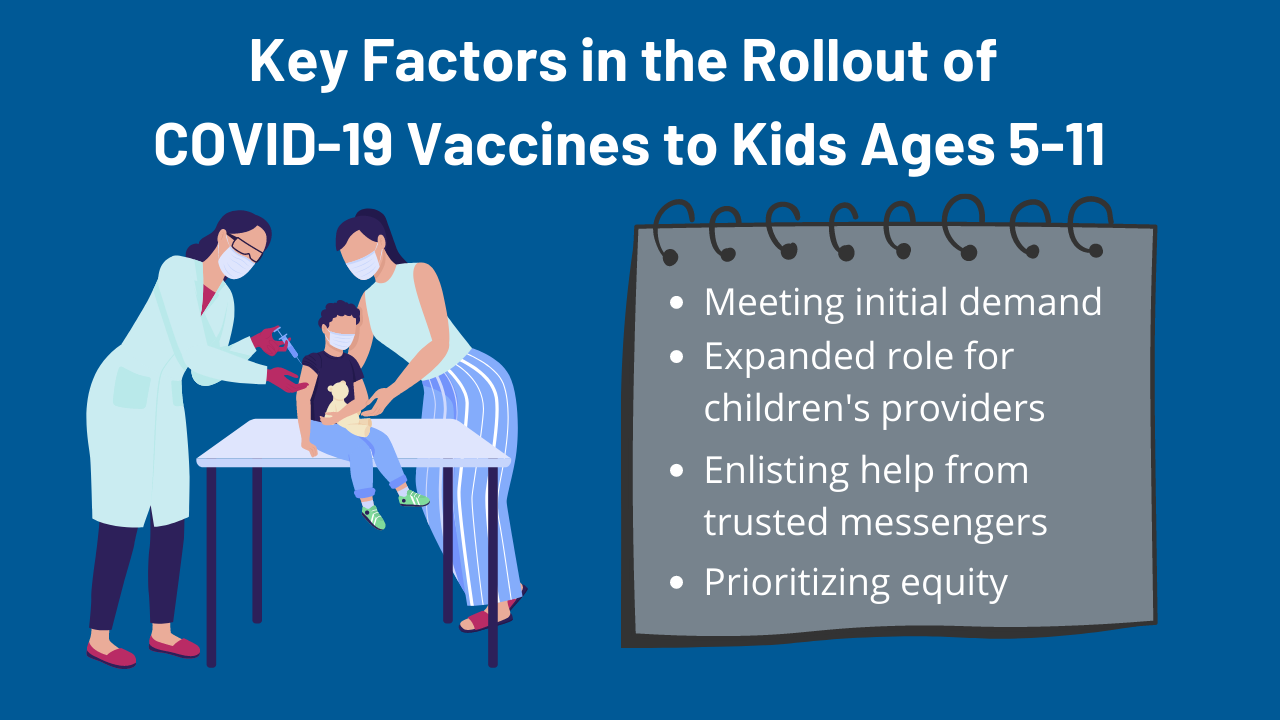
Vaccinating Children Ages 5 11 Policy Considerations For Covid 19 Vaccine Rollout Kff
Kids Covid Vaccine Side Effects What To Know

Babies Children Vaccinate Your Family

Q A Aap Endorses Giving Routine Immunizations Covid 19 Vaccines At Same Time

Covid 19 Vaccine For Kids Under 12 What Parents Need To Know University Hospitals

Covid 19 Moderna Begins Testing Its Vaccine In Children The New York Times

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
:max_bytes(150000):strip_icc()/girlgettingvaccine-e96ea31a3b444b848448a764acf9abc4.jpg)
Pfizer Wins Approval To Add Kids As Young As 12 To Covid 19 Vaccine Trials

When Will A Covid 19 Vaccine Be Ready For Kids Under 12 And What S The Latest News On Clinical Trials Connecticut Children S
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr